PRODUCT TEST RESULTS
MULTIMEDIA PRESENTATION

UPRP P.436477

INTELLIGENT MASK OF VIRUSICIDAL


INTERACTIVE EVIDENCE
OF PRODUCT QUALITY / NORMAL / CLASSIFICATION / USEFULNESS
INTERACTIVE DASHBOARDS
BIOCIDAL PROPERTIES OF INVENTI NANO+ MASK












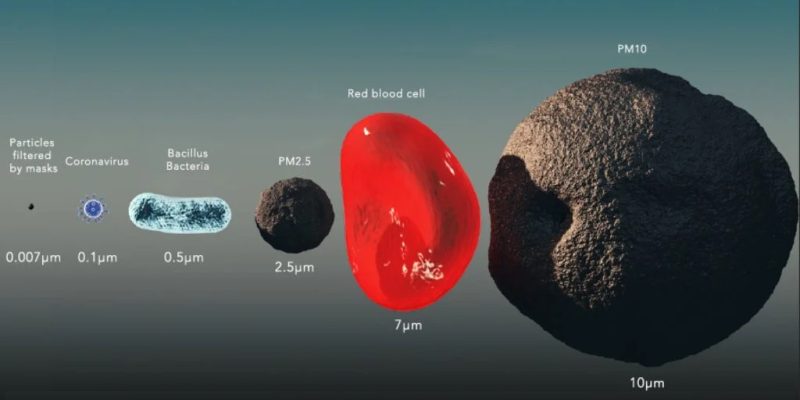
COMPARISON OF ORGANISM SIZES WITH SARS-CoV2
ADDITIONAL STANDARDS FULFILLED BY INVENTI NANO+ MASK








PASSIVE FILTRATION EFFICIENCY FFP2 / FFP3
Masks for personal protective equipment (eg FFP2, FFP3) are tested against the requirements in order to obtain a Passive Particulate Filtration Efficiency (PFE) result and not a Virus Filtration Efficiency (VFE) result.
Test present mask filter are conducted according to standard EN 149: 2001 + A1: 2009 – Test Method NIOSH NaCl aerosol, is dedicated to protect the respiratory tract against solid particles, which results in determining the effectiveness of the passive filtering FFP1 / FFP2 / FFP3.
Above standard is harmonized with the Regulation of the European Parliament and the EU Council No. 2016/425 dated 09.03.2016 year , which regulates the placing of personal protective equipment n and Union market of Euro Union.
In the USA, the CDC (Centers for Disease Control and Prevention) for the national N95 PFR standard also uses a comparable test method used in the European Union. Products tested according to PFR standards, such as N95, are approved on the US market by the “FDA” (US Food & Drug Administration).
The efficiency of passive filtration of FFP for solid particles due to the minimum size of harmful particles in the test aerosol (minimum particle size – 300 nm) does not meet the requirements and the efficiency of filtration of viruses (VFE) , which are much smaller in size.
The SARS CoV2 virus is approximately 100 nm in size , so it is three times smaller than the smallest particle of the aerosol used in the PFE test (FFP1 / FFP2 / FFP3) (minimum size – 300 nm).
Disadvantages of protective masks for solid particles tested with the "PFE" method (FFP1 / FFP2 / FFP3) from the point of view of biocide and virus filtration efficiency.
Major defects masks protective respondents usually only method (PFE) in comparison with the other masks and mask biocidal Inventi Nano+:
- The solid particles used for testing are approximately three times larger than the SARS COV2 virus and thus penetrate the mask to both the wearer and other people in the environment.
- The efficiency of passive particle filtration “PFE” is performed only for solid particles (masks in this standard are not tested with viruses, let alone SARS CoV2).
EFFECTIVENESS OF PASSIVE BACTERIAL FILTRATION "BFE"
AND VIRUSICOCICITY AND HUMAN WELFARE
The immune system fights viruses differently than against bacteria and their toxins that are many times larger.
The first line of defense of the human body against viruses are the so-called cytotoxic cells (NK lymphocytes). Lymphocytes are an element of both anti-infective and anti-tumor cellular immunity.
Bacteria are mainly fought with the use of antibodies that expose bacterial cells to the immune system, which is an element of humoral immunity.
High efficiency FILTER PASSIVE masks type BFE, does not translate into FULL PROTECTION OF HUMAN BEFORE THE VIRUS . The filtration properties of BFE masks only support the process of COMPREHENSIVE PROTECTION of the body by protecting it against contamination with bacterial cells and thus the development of accompanying bacterial diseases.
Medical masks, surgical examined in the bacterial filtration effectiveness passive “BFE” are designed primarily all in order to oh rony sensitive the patient ‘ shapes against threats (and nfekcjami) from the side of employees health . They are stopping wearer (eg. C surgeon) pr zed spread of germs during coughing / sneezing / speech. Areso designed as to protect the patient, and not the user.
Disadvantages of traditional surgical and medical masks tested with the "BFE" method from the point of view of biocidal activity and virus filtration efficiency.
Major defects masks surgical adult respondents usually only method (BFE) in comparison with the other masks and mask biocidal Inventi Nano+:
- Bacteria are at least five times larger than the SARS COV2 virus, and thus penetrate the mask to both the wearer and other people in the environment.
- There is no standard surgical masks closely fit to the face, which leaves a space around the edges m flat.
- The efficiency of passive bacterial filtration “BFE” is performed only for bacterial strains (masks in this standard are not tested with viruses, let alone SARS CoV2).
EFFECTIVENESS OF ACTIVE FILTRATION
OF THE INVENTI NANO+ MASK
TESTED ON FILTRATION EFFICIENCY / VIRUS BIOCIDALITY (VFE)
IS 99.9%
OF THE INVENTI NANO+ MASK
TESTED ON FILTRATION EFFICIENCY / VIRUS BIOCIDALITY (VFE)
IS 99.9%
THE EFFECTIVENESS OF PASSIVE FILTRATION BACTERIAL "BFE"
OF THE INVENTI NANO+ MASK
IS 99.9%
OF THE INVENTI NANO+ MASK
IS 99.9%
COMPLIANCE WITH EUROPEAN STANDARDS ENSURES THE SAFETY AND QUALITY OF MEDICAL DEVICES
MATERIAL

SAFETY CE

CLINICAL TESTS

FILTRATION EFFICIENCY

QUALITY SYSTEM

BIOLOGICAL EVALUATION

RISK MANAGEMENT

APPLICABLE ENGINEERING

MEDICAL INFORMATION

MARKING / LABELLING

COMPARISON OF THE SCOPE OF
TEST / STANDARDIZATION TECHNOLOGY
functionality | inventi | MASK Surgical | Mask Medical | Mask protective | Mask Material |
|---|---|---|---|---|---|
TECHNOLOGY STANDARD | NANO+ | No | No | No | No |
ASSIVE PARTICLE FILTRATION CLASS | FFP3 | Option | Option | Option | No |
CLASS OF PASSIVE BACTERIAL FILTRATION | Type II BFE≥ 99,9% | Type I R BFE≥ 95% | Type I BFE≥ 95% | No | No |
BACTERIAL FILTRATION TEST (0.1 micron particle) VS RESISTANCE (MINIMUM PRESSURE in mm Hg = 160) ASTM F2299 / F2299M-03 FDA (USA) ASTM F2100-19 | LEVEL3 CLASS1 BACTERIAL FILTRATION > 99,9% VS RESISTANCE (pressure in mm Hg) =160 | LEVEL1 ≥95% VS RESISTANCE (pressure in mm Hg= 80) LEVEL2 ≥ 98% VS RESISTANCE = 120 LEVEL3 ≥98% VS RESISTANCE =160 | LEVEL1 ≥95% VS RESISTANCE (pressure in mm Hg= 80) | No | No |
VIRUSOCICIDAL | Yes > 99,9% | No | No | No | No |
BACTERICIDALITY | Yes > 99,9% | No | No | No | No |
FUNGISIDE | Yes > 99,9% | No | No | No | No |
MAINTAINING THE HIGHEST LEVEL OF PROTECTION, BIOCIDALITY - 99.9% | minimum 48h - 72h | No | No | No | No |
NANOTECHNOLOGICAL PRODUCT | Yes | Option | Option | No | No |
MEDICAL PRODUCT | Yes | Yes | Yes | No | No |
NDS OF PARTICLES ("NDS" - - the maximum permissible concentration) | 9xNDS NDS ≥ 0,05mg/m3 | 9xNDS NDS ≥ 0,05mg/m3 | 9xNDS NDS ≥ 0,05mg/m3 | 4xNDS NDS ≥ 0,05mg/m3 | No |
BREATHING COMFORT - EQUALITY OF AIR PRESSURE DISTRIBUTION | Yes | No research / data available | No research / data available | No research / data available | No research / data available |
COUNTERACTING RE-EMISSION OF VIRUSES TO THE BODY | Yes | No | No | No | No |
USE ENVIRONMENT | Human community, health service | Operating room | Health Service | Other | No |
NANOTECHNOLOGY, PASSIVE FILTRATION, ACTIVE SELECTIVE BIOCIDALITY - INVENTI
Quantitative elimination of the virus as a result of passive filtration (BFE / FFP2 / FFP3) is difficult due to the size and kinetics of the SARS CoV2 microorganism.
The nanotechnological activity of the Inventi Nano + coating with the use of the biocidal function released in time is an ACTIVE form of quantitative elimination of viruses, including the SARS CoV2 virus.
On the surface of the fibers of the outer layer of the mask there is a nanotechnological coating, capable of inactivating viruses by contact. The substances contained in the coating, including the iodine-polymer complex, activate the biocidal effect over time, inactivating proteins, fatty acids and, importantly, the GENETIC MATERIAL OF THE VIRUS (RNA) , DESTROYING THE CORONAVIRUS SPINDLE PROTEIN AND THE READY SPIKE (the function mediating penetration into the virus through the ACE2 receptor) cells) (see the video “Infection of cells with SARS CoV2 virus) , making it harmless to humans.
Importantly – as the cell’s ACE2 protein is like a “DOOR LOCK” for a virus ” KEY” (an enzyme called transmembrane serine protease type II / TMPRSS2), the neuropilin-1 protein, which occurs in the nerve tissues of the nasal cavity, is a specific “ROUTE “. Through SELECTIVE ACTIVE FILTRATION USING BIOCIDAL PROPERTIES , a specific ” DAMAGE ” of the virus is made in the reading of information about the ” WAY ” to human cells.
The self-cleaning coating, the active ingredient of which is povidone-iodine, kills most forms of vegetative microorganisms in the FIRST 15-30s . The destructive activity is maintained for a LONGER TIME , thanks to polymer cross-linking, which means that the preparation has, IN THE WHOLE SPECTRUM, PROLONGED BIOCIDAL EFFECTIVENESS (including virus neutralization).
Viruses, in particular SARS CoV2, have a size of about 100 nm (0.1 μm) [1000 μm = 1 mm) / (10 −6 m )] and tests of the PFE passive filtration efficiency (FFP1, FFP2, FFP3) are performed for particles with a minimum 300 nm size – Europe.
IN THE INVENTI MASK A COMBINATION OF PASSIVE FILTRATION (solid particles + bacteria "BFE"),
NANOTECHNOLOGY, ACTIVE AND SELECTIVE BIOCIDALITY MAKES THE TESTED PRODUCT
A NEW SELF- REFERENTIAL INDIVIDUAL CLASS
NANOTECHNOLOGY, ACTIVE AND SELECTIVE BIOCIDALITY MAKES THE TESTED PRODUCT
A NEW SELF- REFERENTIAL INDIVIDUAL CLASS
ENSURING BIOLOGICAL SAFETY
FUNCTIONALITY | INVENTI NANO+ | MASK SURGICAL | Mask MEDICAL | MASK PROTEKTIVE | MASK MATERIAL |
|---|---|---|---|---|---|
BIOSIDAL (> 99,9%) | Yes | No | No | No | No |
PERIOD OF MAINTAINING THE BEST BIOCIDAL EFFECTIVENESS (99.9%) | minimum 48h | No | No | No | No |
REMOVE VIRUSES FROM THE MASK BACK TO SURROUNDINGS | No | Yes | Yes | Yes | Yes |
EMISSION OF VIRUSES FROM THE COATING OF THE MASK TO THE USER'S BODY | No | Yes | Yes | Yes | Yes |
VIRUS EMISSION AFTER CONTACT WITH HANDS | No | Yes | Yes | Yes | Yes |
VIRUS EMISSION IN THE MASK DISPOSAL PROCESS | No | Yes | Yes | Yes | Yes |
DESTRUCTION OF HUMAN BACTERIAL FLORA | No | No | No | No | No research / data available |
BIOLOGICAL SAFETY TESTS | Yes | Option | Option | Option | No |
ANTI-ALLERGY | Yes | Yes | Yes | No | No |
SURFACE STATICITY OF THE MATERIAL | minimum 48h | No research / data available | No research / data available | No research / data available | No research / data available |
PROCESS OF VIRUSICIDAL PROTECTION
INVENTI NANO+ mask is acting antiviral agent , which can be safety TOUCH .
On the surface of a standard mask, viruses and microorganisms accumulate freely in the structures of the material. Thus, they are easily transferred, also to parts of the human body (e.g. hands, mouth, nose, skin), elements of clothing, the environment or back to the air.
The Inventi mask, made in NANO+ technology, KILLS ALL VIRUSES, BACTERIA, MITTE AND MUSHROOMS, thanks to the innovative biocidal outer coating. The Inventi NANO+ mask is safe for humans through the BIOLOGICAL SELF-CLEANING PROCESS.
USE OF THE PRODUCT
WITH BIOCIDAL PROPERTIES
FUNCTIONALITY | INVENTI NANO+ | MASK SURGICAL | Mask MEDICAL | MASK PROTEKTIVE | MASK MATERIAL |
|---|---|---|---|---|---|
WEARING COMFORT | Yes | No research / data available | No research / data available | No research / data available | No research / data available |
BREATHING COMFORT - PARAPPERMEABLE | Yes | Yes | Yes | No research / data available | No research / data available |
BREATHING COMFORT - EQUALITY OF AIR DISTRIBUTION WHEN BREATHING | Yes | No | No | No | No |
BREATHING COMFORT - UNIFORMITY OF AIR SUCTION WHEN INHALED | Yes | No | No | No | No |
USE - HUMAN FOCUS, GROUP OF PEOPLE | Yes | No | Option | No | No |
USE - CLOSED ROOMS | Yes | Yes | Yes | Yes | No |
USE - OUTDOOR USE | Yes | No | No | No | No |
OPERATING TEMPERATURE RANGE | -20 +40 | No research / data available | No research / data available | No research / data available | No research / data available |
PRODUCT WITH INCREASED COMFORT FOR GLASSWARE WEARERS | Yes | No | No | No research / data available | No research / data available |
AGE RANGE OF USERS | Pełen | Pełen | Pełen | Option | No research / data available |
ELIMINATION OF SMOG | Yes | No | No | No | No |
CONTAMINATION ELIMINATION | Yes | No | Option | No | No |
APPLICATION RECOMMENDATIONS
FUNCTIONALITY | INVENTI NANO+ | MASK SURGICAL | Mask MEDICAL | MASK PROTEKTIVE | MASK MATERIAL |
|---|---|---|---|---|---|
"WHO" WORLD HEALTH ORGANIZATION | Yes | Yes | Yes | No | No |
"CDC" CENTER for DISEASE CONTROL and PREVENTION | Yes | Yes | Yes | No | No |
MINISTRY OF HEALTH | Yes | Yes | Yes | No | No |
MEDICAL COUNCIL OF THE RP | Yes | Yes | Yes | No | No |
STATE DEPARTMENT OF HYGIENE | Yes | Yes | Yes | No | No |
LABORATORIES AND HOSPITALS | Yes | Yes | Yes | No | No |
MEDICAL ENVIRONMENT | Yes | Yes | Yes | No | No |
PRODUCT USERS | Yes | Yes | No research / data available | No research / data available | No research / data available |
